Vaccine savings from flu to shingles
Optum Perks offers discounts on the vaccines you and your family may need.

Popular vaccines
COVID-19 vaccines omitted
Use this discount card to save on vaccines today

Use this card to get discounts on most prescriptions at over 64,000 pharmacies nationwide. Each time you fill a prescription, simply show your discount card to your pharmacist.
- Get discounts of up to 80% on most prescription drugs.
- Our prices beat the competition 70% of the time.*
- Get discounts for every member of your family.
- No expiration. No fees or obligations. No credit card required.
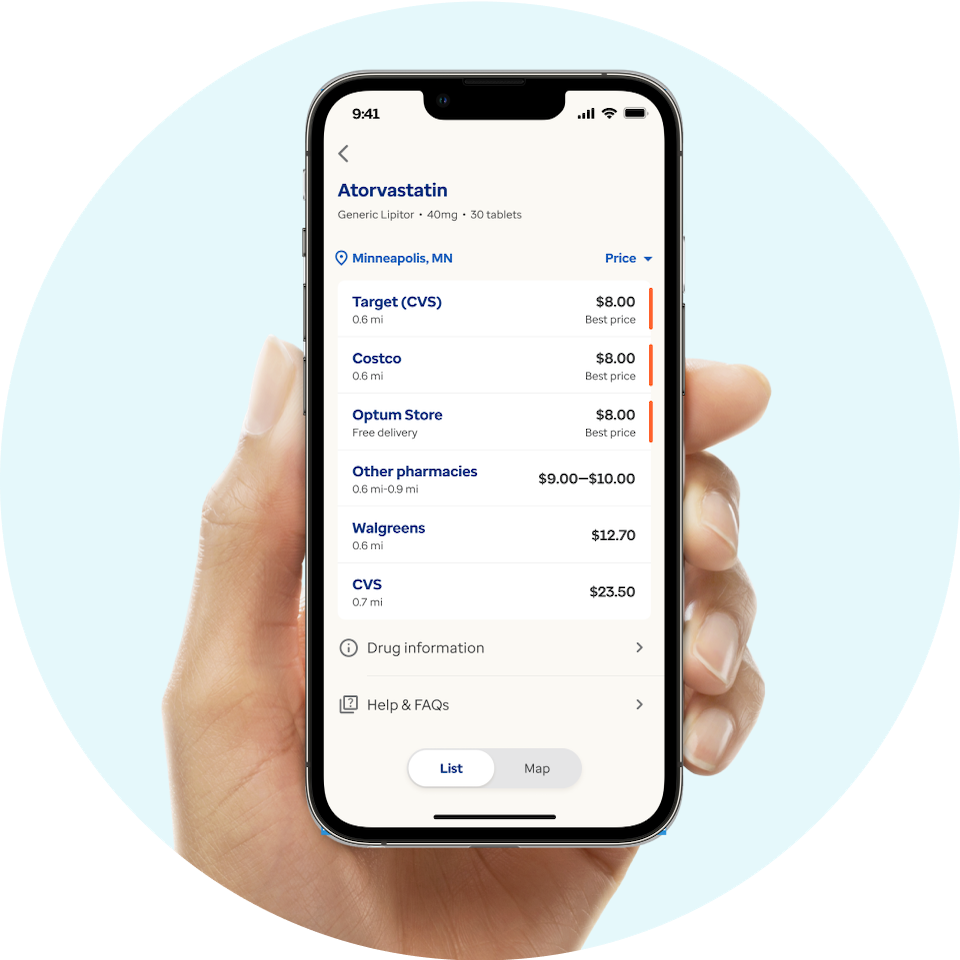
Find the best discounts on prescription drugs anywhere you go.
Get the free Optum Perks app for iOS and Android and start saving today!